This page explains what happens when organic compounds absorb UV or visible light, and why the wavelength of light absorbed varies from compound to compound.
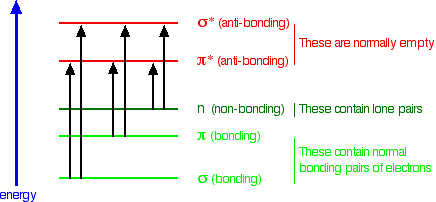
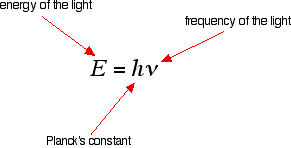
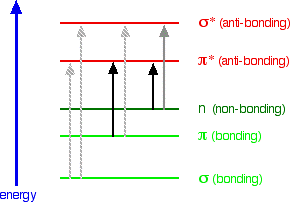
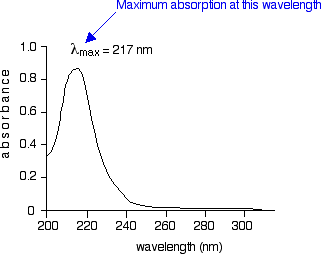
What happens when light is absorbed by molecules? Promotion of electrons When we were talking about the various sorts of orbitals present in organic compounds on the introductory page (see above), you will have come across this diagram showing their relative energies: Remember that the diagram isn't intended to be to scale - it just shows the relative placing of the different orbitals. When light passes through the compound, energy from the light is used to promote an electron from a bonding or non-bonding orbital into one of the empty anti-bonding orbitals. The possible electron jumps that light might cause are: In each possible case, an electron is excited from a full orbital into an empty anti-bonding orbital. Each jump takes energy from the light, and a big jump obviously needs more energy than a small one. Each wavelength of light has a particular energy associated with it. If that particular amount of energy is just right for making one of these energy jumps, then that wavelength will be absorbed - its energy will have been used in promoting an electron. We need to work out what the relationship is between the energy gap and the wavelength absorbed. Does, for example, a bigger energy gap mean that light of a lower wavelength will be absorbed - or what? It is easier to start with the relationship between the frequency of light absorbed and its energy: You can see that if you want a high energy jump, you will have to absorb light of a higher frequency. The greater the frequency, the greater the energy. That's easy - but unfortunately UV-visible absorption spectra are always given using wavelengths of light rather than frequency. That means that you need to know the relationship between wavelength and frequency. You can see from this that the higher the frequency is, the lower the wavelength is. So . . . If you have a bigger energy jump, you will absorb light with a higher frequency - which is the same as saying that you will absorb light with a lower wavelength. Important summary The larger the energy jump, the lower the wavelength of the light absorbed. Some jumps are more important than others for absorption spectrometry An absorption spectrometer works in a range from about 200 nm (in the near ultra-violet) to about 800 nm (in the very near infra-red). Only a limited number of the possible electron jumps absorb light in that region. Look again at the possible jumps. This time, the important jumps are shown in black, and a less important one in grey. The grey dotted arrows show jumps which absorb light outside the region of the spectrum we are working in. Remember that bigger jumps need more energy and so absorb light with a shorter wavelength. The jumps shown with grey dotted arrows absorb UV light of wavelength less that 200 nm. The important jumps are: from pi bonding orbitals to pi anti-bonding orbitals; from non-bonding orbitals to pi anti-bonding orbitals; from non-bonding orbitals to sigma anti-bonding orbitals. That means that in order to absorb light in the region from 200 - 800 nm (which is where the spectra are measured), the molecule must contain either pi bonds or atoms with non-bonding orbitals. Remember that a non-bonding orbital is a lone pair on, say, oxygen, nitrogen or a halogen. Groups in a molecule which absorb light are known as chromophores. What does an absorption spectrum look like The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene - a molecule we will talk more about later. Absorbance (on the vertical axis) is just a measure of the amount of light absorbed. The higher the value, the more of a particular wavelength is being absorbed. You will see that absorption peaks at a value of 217 nm. This is in the ultra-violet and so there would be no visible sign of any light being absorbed - buta-1,3-diene is colourless. You read the symbol on the graph as "lambda-max". In buta-1,3-diene, CH2=CH-CH=CH2, there are no non-bonding electrons. That means that the only electron jumps taking place (within the range that the spectrometer can measure) are from pi bonding to pi anti-bonding orbitals.
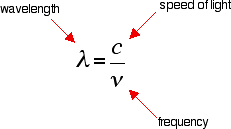
Absorbance
For each wavelength of light passing through the spectrometer, the intensity of the light passing through the reference cell is measured. This is usually referred to as Io - that's I for Intensity.
The intensity of the light passing through the sample cell is also measured for that wavelength - given the symbol, I.
Note: In the classroom we have used the notations Po and P. You can use either notations
If I is less than Io, then obviously the sample has absorbed some of the light. A simple bit of maths is then done in the computer to convert this into something called the absorbance of the sample - given the symbol, A.
For reasons to do with the form of the Beer-Lambert Law (below), the relationship between A (the absorbance) and the two intensities is given by:
A= Log (Io / I )
On most of the diagrams you will come across, the absorbance ranges from 0 to 1, but it can go higher than that. An absorbance of 0 at some wavelength means that no light of that particular wavelength has been absorbed. The intensities of the sample and reference beam are both the same, so the ratio Io/I is 1. Log10 of 1 is zero. An absorbance of 1 happens when 90% of the light at that wavelength has been absorbed - which means that the intensity is 10% of what it would otherwise be. In that case, Io/I is 100/I0 (=10) and log10 of 10 is 1. The importance of concentration The proportion of the light absorbed will depend on how many molecules it interacts with. Suppose you have got a strongly coloured organic dye. If it is in a reasonably concentrated solution, it will have a very high absorbance because there are lots of molecules to interact with the light. However, in an incredibly dilute solution, it may be very difficult to see that it is coloured at all. The absorbance is going to be very low. Suppose then that you wanted to compare this dye with a different compound. Unless you took care to make allowance for the concentration, you couldn't make any sensible comparisons about which one absorbed the most light. The importance of the container shape Suppose this time that you had a very dilute solution of the dye in a cube-shaped container so that the light travelled 1 cm through it. The absorbance isn't likely to be very high. On the other hand, suppose you passed the light through a tube 100 cm long containing the same solution. More light would be absorbed because it interacts with more molecules. Again, if you want to draw sensible comparisons between solutions, you have to allow for the length of the solution the light is passing through. Both concentration and solution length are allowed for in the Beer-Lambert Law. The Beer-Lambert Law What the Law looks like You will find that various different symbols are given for some of the terms in the equation - particularly for the concentration and the solution length. I'm going to use the obvious form where the concentration of the solution is "c" and the length is "b". Log (Io / I ) = a.b.c You should recognise the expression on the left of this equation as what we have just defined as the absorbance, A. You might also find the equation written in terms of A: A= a.b.c The letter 'a' denotes absorptivity.
No comments:
Post a Comment